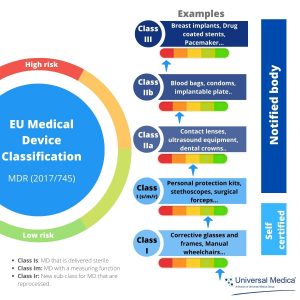
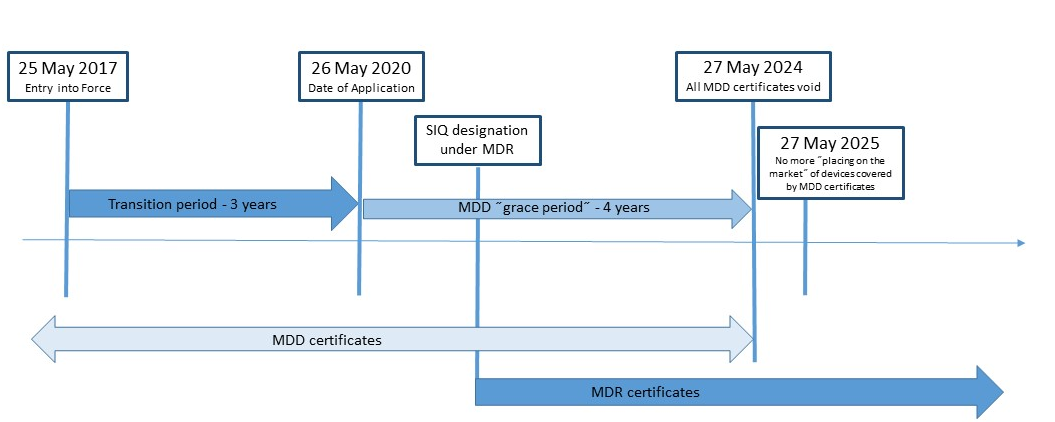
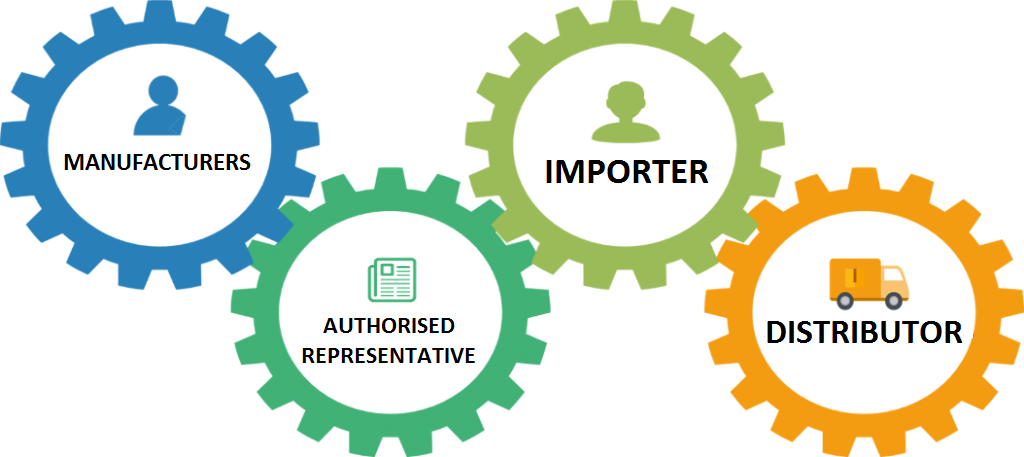
HOW TO BRING A MEDICAL DEVICE TO MARKET IN EUROPE - Leon Research | CRO - Clinical Trials Spain, Italy and Portugal

MEDICAL DEVICE REGULATION (EU) 2017/745: FUNDAMENTAL CHANGES COMPARED TO MEDICAL DEVICE DIRECTIVES (English Edition) eBook : AKDAĞ TATLI, ESRA: Amazon.es: Tienda Kindle

Medical Devices Clinical Evaluation - Summary of Safety and Clinical Performance (SSCP) - Regulation (EU) 2017/745 - GMED Medical Device Certification

EU Medical Device Regulation- Regulation (EU) 2017/745 Of the European Parliament and Of the Council : Council, European, O'Brien, Des: Amazon.es: Libros

Preguntas más frecuentes sobre la nueva Regulación de Dispositivos Médicos de la UE - B Medical Systems (ES)

The EU's Medical Device Regulation (EU) 2017/745 – Are You Ready for Huge Sweeping Changes? - In Compliance Magazine

Safety reporting in clinical investigations of medical devices under the Regulation (EU) 2017/745 | SKYbrary Aviation Safety